Comprehending The Incredible Calcium -Advanced CE
Comprehending The Incredible Calcium
Advanced CE
Author: Mark Parkinson BsPharm: President AFC-CE
Credit Hours 3 - Approximate time required: 180 min.
Educational Goal
Tell why calcium is so important to normal body function.
Educational Objectives
- Explain what calcium is chemically
- Tell how calcium is used in antacids
- Teach about calcium use in bones and teeth
- Tell how calcium is used as an electrolyte in the body
- List sources of calcium
- Talk about calcium supplements
- Discuss calcium pathology
- Quote current scientific studies about calcium
Procedure:
Read the course materials. 2. Click on exam portal [Take Exam]. 3. If you have not done so yet fill in Register form (username must be the name you want on your CE certificate). 4. Log in 5. Take exam. 6. Click on [Show Results] when done and follow the instructions that appear. 7. A score of 70% or better is considered passing and a Certificate of Completion will be generated for your records.
Disclaimer
The information presented in this activity is not meant to serve as a guideline for patient management. All procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this article should not be used by care providers without evaluation of their patients’ Doctor. Some conditions and possible contraindications may be of concern. All applicable manufacturers’ product information should be reviewed before use. The author and publisher of this continuing education program have made all reasonable efforts to ensure that all information contained herein is accurate in accordance with the latest available scientific knowledge at the time of acceptance for publication. Nutritional products discussed are not intended for the diagnosis, treatment, cure, or prevention of any disease.
Comprehending The Incredible Calcium
Advanced CE
We all know about the calcium in our bones and teeth. I wonder though. Do you know just how incredibly important calcium is in the functioning of the rest of our bodies? In this CE lesson, we will discuss that and how calcium does all the incredible things it does. Fair warning though, some of this is going to be advanced stuff. If you don’t want to put in the effort, I have plenty of other CEs to take.
Calcium as an Element
 Calcium has an atomic number of 20 and is an alkaline earth metal. It is the fifth most abundant element in the earth’s crust and is found most often as calcium carbonate. Calcium has many physical and chemical properties that make it one of the most essential minerals for life on this planet. It is very reactive with other chemicals and compounds and dissolves easily in water. When dissolved in water, calcium becomes a chemical ion that carries a strong +2 charge (Ca+2). Because of this strong positive charge, calcium readily combines with other dissolved negatively charged ions like Carbonate (-2 CO3), Phosphate (-3 PO4), Chlorine (-CL), or a variety of protein ions. Because of this spontaneous reactivity, calcium becomes a very useful ingredient in many important biological functions. An example is calcium’s use as an antacid.
Calcium has an atomic number of 20 and is an alkaline earth metal. It is the fifth most abundant element in the earth’s crust and is found most often as calcium carbonate. Calcium has many physical and chemical properties that make it one of the most essential minerals for life on this planet. It is very reactive with other chemicals and compounds and dissolves easily in water. When dissolved in water, calcium becomes a chemical ion that carries a strong +2 charge (Ca+2). Because of this strong positive charge, calcium readily combines with other dissolved negatively charged ions like Carbonate (-2 CO3), Phosphate (-3 PO4), Chlorine (-CL), or a variety of protein ions. Because of this spontaneous reactivity, calcium becomes a very useful ingredient in many important biological functions. An example is calcium’s use as an antacid.
Calcium as an Antacid
 Calcium compounds are often used to neutralize acids. For example, calcium-based antacids are the most common OTC remedy for acidy stomach complaints. Forgive the simple analogy but taking calcium as an antacid is like a singles mixer party. Everyone leaves the date they came with to hang out with someone new. Stomach acid is primarily made up of the compound HCL and antacids are commonly made of Calcium Carbonate. (CaC03). The Calcium (Ca) in the antacid and Chlorine really want to stick to each other. That leaves the acid (H+) and the base (-CO3) to hang out with each other but they really don’t get along very well. Two of the Hydrogen ions steal away one of the Oxygens, and they turn into water (H2O). The leftover CO2 is disgusted with all the breakups and leaves the party as CO2 gas. That’s why you burp when you take an antacid.
Calcium compounds are often used to neutralize acids. For example, calcium-based antacids are the most common OTC remedy for acidy stomach complaints. Forgive the simple analogy but taking calcium as an antacid is like a singles mixer party. Everyone leaves the date they came with to hang out with someone new. Stomach acid is primarily made up of the compound HCL and antacids are commonly made of Calcium Carbonate. (CaC03). The Calcium (Ca) in the antacid and Chlorine really want to stick to each other. That leaves the acid (H+) and the base (-CO3) to hang out with each other but they really don’t get along very well. Two of the Hydrogen ions steal away one of the Oxygens, and they turn into water (H2O). The leftover CO2 is disgusted with all the breakups and leaves the party as CO2 gas. That’s why you burp when you take an antacid.
Overall chemical reaction
CaCO3 (calcium carbonate) + 2HCl (hydrochloric acid) → CaCl2 (calcium chloride) + CO2 (carbon dioxide) + H2O (water)
Acid-Base Neutralization
An Acidic solution is always a concentration of H+ ions. The more H+ ions the stronger the acid. A base always has the ability to give up an -OH ion. When -OH combines with the H+ it makes water. The acid and the base (caustic alkaline) have been neutralized.
The Chemistry
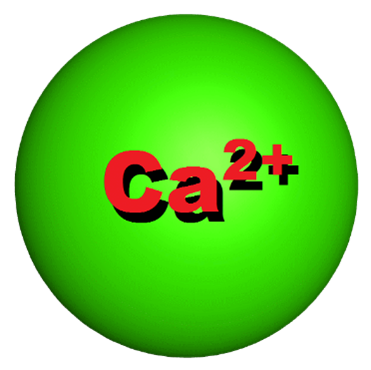 It’s all about the charge. An ion is an atom or molecule with a net electrical charge. Calcium as an element has 20 positively charged protons but only 18 negatively charged electrons. That creates a strong +2 charge overall. When a molecule or other element that has an excess number of electrons comes close, they stick together like magnets and form a crystalline matrix. Following the antacid example above, A carbonate molecule (CO3) has 2 extra electrons, creating a -2 charge. When these two molecules come in contact with each other, they electronically stick together (opposites attract) and form CaCO3. A crystalline substance we know as chalk. When calcium is combined with an anion (a negatively charged ion) the resulting molecules are commonly called calcium salts.
It’s all about the charge. An ion is an atom or molecule with a net electrical charge. Calcium as an element has 20 positively charged protons but only 18 negatively charged electrons. That creates a strong +2 charge overall. When a molecule or other element that has an excess number of electrons comes close, they stick together like magnets and form a crystalline matrix. Following the antacid example above, A carbonate molecule (CO3) has 2 extra electrons, creating a -2 charge. When these two molecules come in contact with each other, they electronically stick together (opposites attract) and form CaCO3. A crystalline substance we know as chalk. When calcium is combined with an anion (a negatively charged ion) the resulting molecules are commonly called calcium salts.
Water (H2O) on the other hand has a weak electric charge because of its V shape. It’s slightly positive on one side and slightly negative on the other. By itself, one molecule of water will not connect stick with either the Ca or the CO3 ion. But if there are a lot of water molecules, the combined partial charge becomes strong enough to pull the matrix apart. Thus, chalk dissolves in water and becomes ions. Now the Calcium and the Carbonate ions are free to be electronically attracted to other ions floating in the water. That’s why we call such ions electrolytes. Calcium is the most abundant mineral in the body. But only 1 % of it is floating around as an electrolyte. Most of the other 99% is locked away in the bones and teeth. More about the 1% later.
Bones and teeth are the hardest substances in our body. We need them to be hard to support all the work and movement we do. So how does an easily dissolvable chemical like calcium provide the support we need without falling apart? Our body combines calcium with other molecules of phosphates and oxides to form a hard-to-dissolve substance called Hydroxyapatite -Ca5(PO4)3(OH). To make it easier to read, I will refer to it as HA from now on. Our bodies take the HA and place it in a matrix surrounded by collagen. The matrix is now a hard yet flexible durable material that will not dissolve by itself or break easily. 65 to 70% of bone mass is HA. Bones are maintained by special cells called osteocytes. Some build up the bone matrix (osteoblasts). Others break down the matrix (osteoclasts). Thus, the bones become a bank account for calcium. When the body comes up short, it can pull it out of the bones.
In teeth, HA makes up 70 to 80% of the dental material. The tooth matrix is made up of HA, amelogenins, and enamelins instead of collagen. Teeth do not have maintenance cells. The acids and other materials in the food we eat and drink can leach out the Calcium and phosphates out of our teeth. The outermost layer is called enamel and is most suspectable to losing its minerals. Fortunately, we can remineralize the enamel with the use of dental preparations. If you include fluorine (like fluorinated water and toothpaste) fluorapatite is incorporated into the enamel instead of HA. Fluorapatite -Ca5(PO4)3F is more resistant to acids than HA is, thus making stronger teeth. Do you see the difference? There is an F instead of an -OH. It will not react the same way with acids. Remember the -OH in the above acid-base reaction.
The Importance of the 1% of Calcium Electrolyte
Of all the calcium in our body only about 1% is floating about in our bodily fluids as an electrolyte. 1% doesn’t sound like much, but that small amount is essential to keeping us alive. Essential Functions of Calcium are:
 It’s a second messenger in cell signaling pathways. (a cascade of events that triggers a certain action in the cell, in the nucleus, or in the cell membrane.
It’s a second messenger in cell signaling pathways. (a cascade of events that triggers a certain action in the cell, in the nucleus, or in the cell membrane.- Plays a role in blood pressure regulation through constriction and relaxation of blood vessels (vasoconstriction and vasodilation)
- It is involved with the electrical transmission in nerve signals.
- Binding calcium ions to proteins creates hormones, enzymes, and various other essential compounds.
- Calcium is involved in releasing the energy needed for muscles to work. That includes the heart muscles.
- Mediates blood clotting
Calcium is incredible. In one way or the other, it has a major effect on every part of our body. However, all those beneficial actions will not happen correctly if the concentration of the free-floating Calcium ion is off. The importance of having the right amount at the right time and in the right place throughout the body cannot be underestimated.
- Too little calcium (hypocalcemia) and body parts won’t work. Calcium is so important that the body will pull it from the bones to get what it needs to function. Even if it makes the bones too weak themselves to function properly.
- Too much calcium (hypercalcemia) and functions get messed up. The body will get rid of the excess to reduce the risk of metabolism failures. The surplus is excreted through the kidneys via the urine and through the intestine within the feces.
- Even within the cells, calcium is strictly regulated.
- The cell has specifically designed channels in the membrane that pump out calcium. (It wants to have more calcium on the outside in the interstitial fluid than on the inside.)
- It also stores away calcium in vesicles to have a ready supply when needed.
So how does the body control the concentration of Calcium? Let’s take a look at the big picture.
Control of the Concentration of Calcium
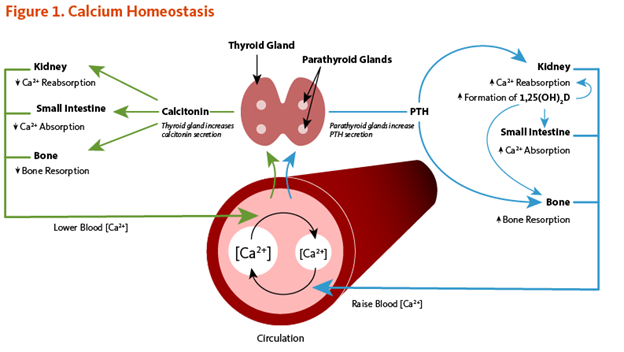
How does the body ensure the right amount of calcium is where it needs to be? The short answer is a negative feedback loop involving parathyroid hormone (PTH), Calcitonin, and Vitamin D. More about that later, but first- let’s look at how we get calcium into our bodies in the first place. Our bodies cannot make calcium. We must get it from the foods we eat. We chew up the food and mix it with liquids that contain digestive enzymes and stomach acids. Remember how water breaks up calcium crystals and acids leach out the calcium ions. Now we have calcium ions floating around in broken-down food mash. The mash flows into the small intestine but the calcium ions can’t easily get into the cells lining the intestinal wall. Calcium needs some help getting past this barrier. That’s where Vitamin D gets involved. The active form of vitamin D (Calcitriol) stimulates the intestinal cells to make special channels in the cell membrane. These channels draw in the calcium from the gut. Then it passes through the cell and into the bloodstream. Now the parathyroid hormone and Calcitonin take over. PTH facilitates calcium entering into cells and Calcitonin inhibits the process.
Now let’s get into the details. This is some of the advanced stuff I warned you about. To make it easier for you, I want you to watch a video. It was made to train nursing students. There will be a few exam questions about the information found in the YouTube video. Don’t worry though. They’re very easy questions to answer.
Calcium in Cellular Actions
We have been talking about the big picture. Now let us focus on the little picture. All the way down to the cellular level. The concentration of calcium ions is always greater on the outside of the cell. Why? Because calcium ions often act like the start button for cellular reactions. You only let the calcium in when you need that action to start. For example,
“Excitable cells, such as skeletal muscle and nerve cells, contain voltage-dependent calcium channels in their cell membranes that allow for rapid changes in calcium concentrations. For example, when a nerve impulse stimulates a muscle fiber to contract, calcium channels in the cell membrane open to allow calcium ions into the muscle cell. Within the cell, these calcium ions bind to activator proteins, which help release a flood of calcium ions from the storage vesicles of the endoplasmic reticulum (ER) inside the cell. The binding of calcium to the protein troponin-c initiates a series of steps that lead to muscle contraction. The binding of calcium to the protein calmodulin activates enzymes that break down muscle glycogen to provide energy for muscle contraction. Upon completion of the action, calcium is pumped outside the cell or into the ER until the next activation.”
Source: https://lpi.oregonstate.edu/mic/minerals/calcium#osteoporosis-prevention
All of this action is driven by the strong +2 charge on the calcium ion. Like magnets, the ions are being pulled together or pushed apart by the ionic charge. This enables the calcium ions to:
- Travel against the flow of fluids.
- Move from areas of high concentrations to low concentrations.
- Be connected to proteins, thus stabilizing them.
- Fill cellular receptors (pushing the on button).
Unfortunately, cellular concentrations and ionic combinations are not all good news. Things can go wrong.
- There are many calcium-dependent processes within the cell. If calcium concentrations remain too high in the cell for too long, all sorts of things go wrong, and the cell dies. That’s called cell apoptosis.
- If Calcium, Vitamin D, or Parathyroid hormone levels get messed up, those things lead to Hypercalcemia and Hypocalcemia. Too much and too little calcium in the blood. Muscles don’t work right, nerves don’t fire well, constipation, etc…, not a very pleasant experience.
- Also, the same ionic actions can lead to molecules that are unwanted. Calcium salts and HA crystals can build up in areas we don’t want them to be. Joints can become ossified, (become like bone), blood vessels can become hardened, and crystals can form in or on tumors, implanted medical devices, glands, and organs. An example is kidney stones. Brain structures can also collect crystals commonly known as brain sand.
To make it all simple. Controlling that 1% ionic calcium concentration is very important.
Well, that’s enough of that. Let’s talk about something more pleasant, FOOD.
Sources of Calcium
Healthy babies start out with about 26 to 30 grams of calcium. As the child grows the amount rises quickly. It peaks at about 1,200 g in women and 1,400 g in men by adulthood. Calcium levels remain constant in men, but they start to drop in women as a result of increases in bone remodeling. (During menopause, the loss of estrogen affects the action of bone maintenance cells, resulting in a net loss of HA.)
So where does all that calcium come from? You know the answer- Food. In American diets, 75% of the calcium we take in comes from dairy products.
Amounts of calcium in common foods
- 8-ounce glass of milk = 300 mg
- 8-ounce glass of calcium-fortified soy milk = 300 mg
- 5 ounces of cheese = 300 mg
- 6 ounces of yogurt = 300 mg
- 3 ounces of sardines with bones = 300 mg
- ½ cup of cooked turnip greens = 100 mg
- ¼ cup of almonds = 100 mg
- 1 cup of shredded bok choy = 74 mg
- 1/4 cup of dried figs = 61 mg
- 6 in. Corn tortilla = 46 mg
 ½ pinto beans = 39 mg
½ pinto beans = 39 mg- ½ cup broccoli = 21mg
Calcium is a listed item in the nutritional Facts that are required on food packaged and sold in America. Consumers can see for themselves how much calcium is in the foods they eat.
Knowing which foods contain calcium and actually getting enough calcium inside you are two different things. It is a much more complicated process than what you might think.
First: How much calcium is needed?
Dietary Reference Intakes for calcium:
- Infants:
0 to 6 months: 200 milligrams per day (mg/day)
7 to 12 months: 260 mg/day
- Children and adolescents:
Age 1 to 3: 700 mg/day
Age 4 to 8: 1,000 mg/day
Age 9 to 18: 1,300 mg/day
- Adults:
Age 19 to 50: 1,000 mg/day
Age 51 to 70: Men - 1,000 mg/day; Women - 1,200 mg/day
Over age 70: 1,200 mg/day
- Pregnancy and breastfeeding:
Age 14 to 18: 1,300 mg/day
Age 19 to 50: 1,000 mg/day
Source: https://medlineplus.gov/ency/article/002412.htm
Second: Not all foods have the same calcium bioavailability.
The calcium is there in the food but it’s harder for the body to absorb it. Dairy is the best but-
“The calcium content in calcium-rich plants in the kale family (broccoli, bok choy, cabbage, mustard, and turnip greens) is as bioavailable as that in milk; however, other plant-based foods contain components that inhibit the absorption of calcium. Oxalic acid, also known as oxalate, is the most potent inhibitor of calcium absorption and is found at high concentrations in spinach and rhubarb and somewhat lower concentrations in sweet potatoes and dried beans. Phytic acid (phytate) is a less potent inhibitor of calcium absorption than oxalate. Yeast possesses an enzyme (phytase) that breaks down phytate in grains during fermentation, lowering the phytate content of breads and other fermented foods. Only concentrated sources of phytate, such as wheat bran or dried beans, substantially reduce calcium absorption” Source: https://lpi.oregonstate.edu/mic/minerals/calcium#osteoporosis-prevention
Third: Vitamin D and other medicines affect how much calcium is absorbed leading to variability.
Fourth: Diets don’t exist in a bubble. What we eat on a consistent basis is influenced by several factors. Food allergies, taste preferences, cooking skills, food costs, culture, and even religion all determine what foods we consume. Wrapping a menu solely around the need for calcium is not very sustainable.
It is a small wonder that up to 46 % of Americans do not get the calcium they need in their diets. Many people turn to calcium supplements to make up the difference.
How to get more Calcium
Getting more calcium to supplement the diet can be done in several different ways.
- Calcium fortified food. Manufacturers of foodstuffs purposely add extra calcium to their products. For example- Wheat is not a good source of calcium, but Cream of Wheat in 1-1/4 cups of milk contains 630 mg (50% of the Recommended Daily Allowance (RDA) for calcium). Oats do not contain calcium but Cheerios in ¾ cup of skim milk contain 25% RDA.
- Medical/Nutritional preparations contain calcium.
Antacids
-
- Tums Regular Strength: 200 mg of elemental calcium per tablet
- Tums Extra Strength: 300 mg of elemental calcium per tablet
- Tums Ultra Strength: 400 mg of elemental calcium per tablet
Multivitamins
-
- Centrum Silver contains 230 mg of calcium per tablet.
Meal replacement drinks.
-
- Ensure Shakes contain 330 mg of calcium per dose.
- Calcium Supplements
Calcium supplements come in many different formulations. They can be purchased as tablets, capsules, chews, liquids, and powders. Combined with Vitamin D and other minerals, (most often combined with Magnesium). They also come in a variety of different salts including calcium carbonate, calcium citrate, calcium citrate malate, calcium lactate, and calcium gluconate.
Calcium Supplements
Calcium preparations come in a great variety of choices. Choosing which is best depends on several different factors and is determined by the needs of the patient. The doctor knows this, but the details may get lost when considering all the other factors involved. You as the caregiver may have to point out the important details to the doctor or his staff.
Cost-
Calcium carbonate tablets are the least expensive formulation therefore the one preferred by insurance. To get a more expensive variety would have to be justified by patient circumstances.
Ease of Consumption-
Calcium is a bulky element. Putting enough calcium in each tablet makes them large and harder to swallow. If the patient has difficulty swallowing or refuses tablets then capsules, chews, liquids, or powders may be justified.
Side effects-
The main side effect of calcium is constipation. Adding magnesium which can cause diarrhea can help counteract constipation. Gas and bloating may also become an issue. Recall the calcium carbonate and acid reaction mentioned previously. Other calcium salts may have less gaseous effects.
Stomach Acid-
Calcium carbonate is dependent on stomach acid for absorption, Calcium Citrate and Calcium Malate do not. Conditions that affect stomach acid might justify the added cost of the citrate or malate. Those conditions include:
Chron’s disease, Irritable Bowel Syndrome
Naturally lower levels of stomach acid production (achlorhydria)
Chronic ulcers and or GERD that require acid blocker therapy
Excessive bloating
Vitamin D-
Adding vitamin D does not add much to the cost and is generally well accepted by insurance. Whether vitamin D is needed depends on the patient’s exposure to sunlight in sufficient quantities.  The active form of Vitamin D is calcitriol (D3). Look for the D3 on the label.
The active form of Vitamin D is calcitriol (D3). Look for the D3 on the label.
When to take calcium for best results
Since calcium carbonate relies on acid, taking it with food or at mealtimes helps ensure the presence of stomach acid. In addition, only so much calcium can be absorbed at a given time. Excess amounts will just pass through and be excreted in the feces. It is recommended to take 500-600 mg from all sources at a time. This may require taking supplements twice daily.
When Things Go Wrong
Normally, In-Home caregivers don’t have to worry too much about calcium levels in their residents. Their bodies do a pretty good job taking care of themselves. There are a few things that you do need to keep an eye on though.
Osteoporosis
Adult Foster care clients are at greater risk of chronic bone loss that leads to fractures and broken bones. Sometimes the mere act of standing up will break a leg. Before they moved into your home there may have been years of poor calcium and bone maintenance. Not enough exercise to stimulate increased bone mass. Avoiding exposure to the sun leads to not enough vitamin D. Not enough Calcium in the diet. Chronic use of medications that interfere with calcium. Eventually, the resident is going to get overdrawn at the bone calcium bank. Fortunately, all you have to do is periodically ask the Doctor for a Bone density test for the resident. The doctor can take it from there.
Dehydration
The thirst drive in your residents, especially the elderly, may be reduced. This leads to chronic dehydration. If there is not enough water in the blood and tissues, ionic calcium can get messed up. Remember the water is what pulls apart the calcium salts. To keep an eye on things by looking at how dry and flakey the skin looks. You can also pinch the skin on the hand or arm. If it bounces back normally then you’re okay. If not---more water please.
Broken Control Mechanisms
Thyroid surgery, tumors, cancer, chronic intestinal problems like IBS, kidney disease, and blood pH problems can all interfere with calcium homeostasis. If your clients have any of these problems, ask the doctor to include a calcium blood test in the regularly scheduled exams. You can also watch for the classic signs of blood calcium problems. Chronic constipation, slow muscle reactions, lower mental acuity, seizures, muscle twitches, abdominal pain, muscle cramps, tingling in the hands and feet, and changes in urination. If these become problematic, ask for that blood test.
Eating Disorders
Mental Health homes with eating disorder patients and Developmentally Disabled homes with extremely finicky eaters may have problems with dietary calcium intake.
Drug Interference
 Many drugs can interact with calcium. I’ll make this short and simple.
Many drugs can interact with calcium. I’ll make this short and simple.
- Thiazide diuretics (water pills like HTCZ) can cause the kidney to absorb more calcium back into the blood. Watch for hypercalcemia.
- Lanoxin (digoxin) and calcium supplements can lead to heart arrhythmias.
- Calcium may decrease the absorption of tetracycline, quinolone class antibiotics (Cipro), bisphosphonates (osteoporosis meds), sotalol (a β-blocker), and levothyroxine. You should separate them and calcium-rich foods and supplements by two hours at least.
- Calcium supplements can decrease the effectiveness of some HIV drugs. Again, separate them by 2 hours.
- H2 blockers (cimetidine) and proton-pump inhibitors (omeprazole) may decrease calcium absorption.
- Lithium increases the risk of hypercalcemia.
- Topical calcipotriene, a vitamin D analog, in the treatment of psoriasis may cause hypercalcemia. As what you might expect knowing what you know about vitamin D and calcium.
- Magnesium has a connection with calcium. For example, too much magnesium (chronic Milk of Magnesium use) or low magnesium blood levels will mess with calcium concentrations.
- Steroids and Corticosteroids when used chronically; like in some asthma inhalers (Flovent, Pulmicort) can lead to bone mass loss. No bone, no calcium bank.
- You normally don’t have to worry about the calcium channel blocker class of high blood pressure medicine and calcium. The name might make you think otherwise but they are okay together.
There are computer programs that tell the doctor and the pharmacist that a drug interaction may occur. Drug interactions are not black and white though. There are a lot of other factors involved that determine if there is a risk for adverse events occurring. The doctor and pharmacist know of the drug interactions but have determined that the benefits out way the risks. If harmful symptoms start occurring in the patient the doctor would probably alter the prescribed therapy. That is where you come in. You are the eyes and ears of the doctor. Watch for the classic calcium problem symptoms if your residents take any of the above meds. Then report them to the doctor promptly. Remember you don’t have to diagnose any of the problems above; you just have to suspect they are occurring. If nothing happens and the symptoms become problematic, then get the resident to the doctor and start asking the right questions.
- Is this symptom a sign of a drug interaction between this med and that calcium source?
- Does this problem that is cropping up outweigh the benefits?
- This symptom is starting to be a real problem, is it time to consider altering the therapy?
Cutting Edge Knowledge (Studies)
Medical research on the effects of calcium on the human body is ongoing. New hypotheses are always being explored. You as a medical care provider can be a part of that effort by observing and reporting on the things you see.
The following is more advanced stuff. If you have difficulty reading and understanding the material, it’s okay. There is only 1 easy exam question covering this material. It is kind of exciting though to be part of this work, even if it’s only a very small part.
Source for items in quotations. Calcium | Linus Pauling Institute | Oregon State University
Zinc
“Although high calcium intakes have not been associated with reduced zinc absorption or zinc nutritional status, an early study in 10 men and women found that 600 mg of calcium consumed with a meal halved the absorption of zinc from that meal”
Cardiovascular Disease
“Several observational studies and randomized controlled trials have raised concerns regarding the potential adverse effects of calcium supplements on cardiovascular risk. The analysis of data from the Kuopio Osteoporosis Risk Factor and Prevention (OSTPRE) prospective study found that users of calcium supplements amongst 10,555 Finnish women (ages 52-62 years) had a 14% greater risk of developing coronary artery disease compared to non-supplement users during a mean follow-up of 6.75 years. The prospective study of 23,980 participants (35-64 years old) of the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition cohort (EPIC-Heidelberg) observed that supplemental calcium intake was positively associated with the risk of myocardial infarction (heart attack) but not with the risk of stroke or cardiovascular disease (CVD)-related mortality after a mean follow-up of 11 years (154). Yet, the use of calcium supplements (≥400 mg/day vs. 0 mg/day) was associated with an increased risk of CVD-related mortality in 219,059 men, but not in 169,170 women, included in the National Institute of Health (NIH)-AARP Diet and Health study and followed for a mean period of 12 years. CVD mortality in men was also found to be significantly higher with total (dietary plus supplemental) calcium intakes of 1,500 mg/day and above.”
Hypertension
“The relationship between calcium intake and blood pressure has been investigated extensively over the past decades. A meta-analysis of 23 large observational studies conducted in different populations worldwide found a reduction in systolic blood pressure of 0.34 millimeters of mercury (mm Hg) per 100 mg of calcium consumed daily and a reduction in diastolic blood pressure of 0.15 mm Hg per 100 mg calcium (113). In the DASH (Dietary Approaches to Stop Hypertension) study, 549 people were randomized to one of three diets for eight weeks: (1) a control diet that was low in fruit, vegetables, and dairy products; (2) a diet rich in fruit (~5 servings/day) and vegetables (~3 servings/day); and (3) a combination diet rich in fruit and vegetables, as well as low-fat dairy products (~3 servings/day) (114). The combination diet represented an increase of about 800 mg of calcium/day over the control and fruit/vegetable-rich diets for a total of about 1,200 mg of calcium/day. Overall, the reduction in systolic blood pressure was greater with the combination diet than with the fruit/vegetable diet or the control diet. Among participants diagnosed with hypertension, the combination diet reduced systolic blood pressure by 11.4 mm Hg and diastolic pressure by 5.5 mm Hg more than the control diet, while the reduction for the fruit/vegetable diet was 7.2 mm Hg for systolic and 2.8 mm Hg for diastolic blood pressure compared to the control diet. This research suggested that calcium intake at the recommended level (1,000-1,200 mg/day) may help prevent and treat moderate hypertension.
Yet, two large systematic reviews and meta-analyses of randomized controlled trials have examined the effect of calcium supplementation on blood pressure compared to placebo in either normotensive or hypertensive individuals (117, 118). Neither of the analyses reported any significant effect of supplemental calcium on blood pressure in normotensive subjects. A small but significant reduction in systolic blood pressure, but not in diastolic blood pressure, was reported in participants with hypertension. Of note, calcium supplementation in these randomized controlled trials ranged from 400 to 2,200 mg/day, with 1,000 to 1,500 mg/day being the more common dosages. A more recent meta-analysis of 13 randomized controlled studies in 485 individuals with elevated blood pressure found a significant reduction of 2.5 mm Hg in systolic blood pressure but no change in diastolic blood pressure with calcium supplementation (119). The modest effect of calcium on blood pressure needs to be confirmed in larger, high-quality, well-controlled trials before any recommendation is made regarding the management of hypertension. Finally, a review of the literature on the effect of high-calcium intake (dietary and supplemental) in postmenopausal women found either no reduction or mild and transient reductions in blood pressure.”
Premenstrual Syndrome (PMS)
“PMS refers to a cluster of symptoms, including but not limited to fatigue, irritability, moodiness/depression, fluid retention, and breast tenderness, that begins sometime after ovulation (mid-cycle) and subsides with the onset of menstruation (the monthly period) (100). A severe form of PMS called premenstrual dysphoric disorder (PMDD) has been described in 3%-8% of women of childbearing age. PMDD interferes with normal functioning, affecting daily activities and relationships.
Low dietary calcium intakes have been linked to PMS in early reports, and supplemental calcium has been shown to decrease symptom severity (102). A nested case-control study within the Nurses' Health Study II (NHS II) found that women in the highest quintile of dietary (but not supplemental) calcium intake (median of 1,283 mg/day) had a 30% lower risk of developing PMS compared to those in the lowest quintile (median of 529 mg/day). Similarly, women in the highest versus lowest quintile of skim or low-fat milk intake (≥4 servings/day vs. ≤1 serving/week) had a 46% lower risk of PMS. In a randomized, double-blind, placebo-controlled clinical trial of 466 women with moderate-to-severe premenstrual symptoms, supplemental calcium (1,200 mg/day) for three menstrual cycles was associated with a 48% reduction in total symptom scores, compared to a 30% reduction observed in the placebo group (104). Similar positive effects were reported in earlier double-blind, placebo-controlled, cross-over trials that administered 1,000 mg of calcium daily (105, 106). Recent small randomized controlled trials also reported that supplemental calcium (400-500 mg/day) for three weeks to three months reduced severity and/or frequency of symptoms in women with mild-to-moderate PMS (107-110). Currently available data indicate that daily calcium intakes from food and/or supplements may have therapeutic benefits in women diagnosed with PMS or PMDD.”
Are you as exhausted as I am? That was a lot of material that was covered. Who knew that simple calcium, the stuff that chalk and antacids are made of, is so important to the body? It seemed that calcium affects just about everything. Hopefully, by conquering this advanced material you are now better prepared to be an advanced caregiver.
As always
Good Luck in your Caregiving Efforts
Mark Parkinson BsPharm
References:
- Calcium Fact Sheet for Health Professionals. National Institutes of Health (NIH) Office of Dietary Supplements. July 24, 2024. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Calcium Fact Sheet for Consumers. National Institutes of Health (NIH) Office of Dietary Supplements. Sept 14, 2023. https://ods.od.nih.gov/factsheets/Calcium-Consumer/#:~:text=Calcium%20is%20a%20mineral%20your,giving%20them%20structure%20and%20hardness.
- Calcium and calcium supplements: Achieving the right balance. MayoClininc.org. Nov. 01, 2022. https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/calcium-supplements/art-20047097
- Calcium. Wikipedia, The Free Encyclopedia. Nov 14 2024. https://en.wikipedia.org/wiki/Calcium
- Calcium and Vitamin D. Bone Health and Osteoporosis.org 5/23/2023. https://www.bonehealthandosteoporosis.org/patients/treatment/calciumvitamin-d/
- Beth Kitchin. UAB Tone Your Bones: How Much Calcium Do I Need?. University of Alabama at Birmingham. Mar 31, 2020. https://www.youtube.com/watch?v=HgYsy6kh3eM&t=510s
- Calcium. Linus Pauling Institute, Oregon State University, Sept 2017. https://lpi.oregonstate.edu/mic/minerals/calcium#osteoporosis-prevention
- Calcium. Harvard T.H.Chan School of Public Health Mar 2023. https://nutritionsource.hsph.harvard.edu/calcium/
- Stephanie Watson. Calcium: What You Should Know. WebMD.com. Aug 21, 2024. https://www.webmd.com/vitamins-and-supplements/calcium
- Calcium. Mount Sinai.org. 2024. https://www.mountsinai.org/health-library/supplement/calcium
- Calcium. Wikipedia The Free Encyclopedia. Nov 14 2024. https://en.wikipedia.org/wiki/Calcium
- Hydroxyapatite. Wikipedia The Free Encyclopedia. Nov 30 2024.https://en.wikipedia.org/wiki/Hydroxyapatite
- Endocrinology | Parathyroid Gland | Calcitonin. Ninja Nerd. Medical Lecturers. May 11, 2017. https://www.youtube.com/watch?v=y64aXKCkHk4&t=299s
- Joanne Lewsley, et al. 7 Best Calcium Supplements for 2024. Medical News Today.com, Api 18, 2024. https://www.medicalnewstoday.com/articles/best-calcium-supplements#faq
Exam Portal
click on [Take Exam]
Purchase membership here to unlock Exam Portal.
|
|
|
|
|
*Important*
Registration and login is required to place your name on your CE Certificates and access your certificate history.
Username MUST be how you want your name on your CE Certificate.